New Criteria for Alzheimer Disease and Mild Cognitive Impairment
Implications for the Practicing Clinician
Neurologist. 2012;18(6):356-363.
Abstract
Background: In most research studies and clinical trials, Alzheimer disease (AD) has been diagnosed using the criteria developed by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association work group in 1984. Developments over the last 27 years have lead to the need for new diagnostic criteria.
Review Summary: Four articles in the journal Alzheimer's & Dementia in 2011 describe new criteria for AD dementia and mild cognitive impairment (MCI) due to the AD pathophysiological process (MCI due to AD) and the underlying rationale for them. These new criteria emphasize that the AD pathophysiological process starts years and perhaps decades before clinical symptoms, and that biomarkers can be used to detect amyloid β deposition and the effects of neurodegeneration in the brain.
Conclusions: These new criteria are immediately helpful to the practicing clinician, providing more accurate and specific guidelines for the diagnosis of AD dementia and MCI due to AD. As new diagnostic tools and new treatments for AD become available, diagnosis using these criteria will enable patients with this disorder to receive the best possible care
Rationale for New Criteria
Updating the prior criteria developed by the National Institute of Neurological and Communicative Disorders and Stroke and theAlzheimer's Disease and Related Disorders Association work group in 1984, new clinical criteria have recently been published for Alzheimer disease (AD) and mild cognitive impairment (MCI) due to the AD pathophysiological process (MCI due to AD). Four articles in the journal Alzheimer's & Dementia in 2011 describe these criteria and the underlying rationale for them. The first is an introduction that provides the background of why new criteria are needed.[1] The second details the hypothesis that the AD pathophysiological process starts years and perhaps decades before the onset of clinical symptoms and dementia. It also discusses the concept of "preclinical" AD.[2] The third considers both clinical and research criteria for MCI due to AD.[3] Finally, the fourth describes new clinical and research criteria for AD.[4] We will focus on new clinical criteria, the area that will be of greatest use to practicing clinicians, and briefly touch on the research criteria and theoretical issues.
As discussed in these articles, the new criteria were developed because of the recognition of a number of important factors that have changed since 1984:
The overall purpose of these changes to the criteria are to help the clinician identify and treat the disease as early as possible—ultimately before the onset of decline with the goal of enabling individuals to live long and productive lives free of the dementia caused by AD. Recent data from an Alzheimer's Association report suggest that a delay in the onset of symptoms of AD dementia by 5 years would result in a 45% reduction in the number of people with the disorder by the year 2050 and reduce projected Medicare costs of disease from 627 to 344 billion dollars.[5]
In general, the new criteria differ from the original criteria along 2 important dimensions that have both immediate and future implications for the practicing clinician. One is the recognition of 3 stages of AD progression, with the first (preclinical AD) occurring before changes in cognition and everyday activities are detected, the second (MCI due to AD) occurring when cognitive symptoms emerge but function is still relatively unimpaired, and the third (AD dementia) diagnosed when day-to-day function is impaired. By contrast, the original guidelines only diagnosed AD dementia. Another is the incorporation of biomarkers into the diagnosis, whereas the original criteria were based primarily on clinical judgment.
The 3 stages of AD discussed are:
- Preclinical AD: Preceding any clinical changes, this stage is defined by measureable changes in biomarkers and poor performance on challenging cognitive tests. The biomarkers may be detectable by brain imaging or by molecular changes in cerebrospinal fluid (CSF). There are currently no clinical criteria to make the diagnosis of preclinical AD. The primary purpose of the designation of preclinical AD is to provide a framework for researchers to better define this stage of AD.
- MCI due to AD: This stage is characterized by the first clinical changes. Mild changes in memory and other cognitive abilities that are noticeable to patients and families and can be detected through careful evaluation, but are not sufficient to interfere with day-to-day activities.
- Dementia due to AD: This stage is characterized by changes in 2 or more aspects of cognition and behavior that interfere with the ability to function in everyday life.
Preclinical AD and Biomarkers
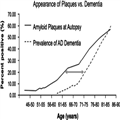
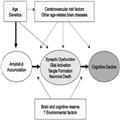
As described by Sperling et al,[2] one commonly used model of AD is that many factors including age, genetics, the environment, and others combine to lead to the accumulation of amyloid β (Aβ) in the brain, which in turn produces synaptic dysfunction, neurofibrillary tangle formation, and neuronal death, all of which ultimately lead to cognitive decline (Fig. 2). Given that the events in this pathologic cascade sequence likely take years or decades from the accumulation of Aβ to the development of dementia or even MCI, there must be a prior "preclinical" stage of AD (Fig. 3).The concept of a preclinical phase of a disease should not be new to practicing clinicians, nor should the concept of diagnosing a disease in the absence of symptoms. Cancer is routinely detected at the stage of "carcinoma in situ" and atherosclerosis may cause detectable narrowing of the arteries before myocardial infarct. Similarly, many diseases including Type II diabetes, osteoporosis, and hypertension are routinely detected and treated before symptoms emerge. The goal is to move AD to a similar status.These guidelines are primarily aimed at helping researchers determine which signature biological changes (ie, biomarkers) occur years before the onset of clinical symptoms. Currently AD biomarkers have minimal impact on clinical practice, although it is likely that these biomarkers will be important to the clinician in the near future.Table 1 presents several biomarkers of Aβ deposition or neurodegeneration that are currently in use. Markers of Aβ deposition include low CSF Aβ42 and positive PET amyloid imaging. Markers of neurodegeneration include elevated CSF tau [both total and hyperphosphorylated tau (p-tau)], decreased metabolism in temporal and parietal cortex on 18flurodeoxyglucose (FDG) PET, and atrophy on magnetic resonance imaging (MRI) in temporal (medial, basal, and lateral) and medial parietal cortex. Although it makes the most sense scientifically to divide the biomarkers into those measuring either Aβ deposition or neurodegeneration, in clinical practice one tends to divide the biomarkers by how they are obtained: structural MRI, PET, and CSF studies.Structural MRI, when analyzed using a quantitative protocol, can provide whole brain volume and volume in anatomically distinct areas of the brain. Patients in whom the AD pathophysiological process has caused neurodegeneration should have less brain tissue in temporal (medial, basal, and lateral) and medial parietal cortex—and they should lose tissue faster—than healthy individuals.[6] Volumetric MRI is currently being used in a number of ongoing clinical trials to evaluate the efficacy of disease-modifying drugs in AD. In addition, the Alzheimer's Disease Neuroimaging Initiative also includes serial volumetric MRIs in AD and MCI patients and healthy controls.[7] The Alzheimer's Disease Neuroimaging Initiative data should ultimately provide a normative database that would be an important step in allowing volumetric MRI to be used clinically. Volumetric MRI analyses are not routinely available, although we encourage all clinicians to look for qualitative patterns of atrophy in temporal (medial, basal, and lateral) and medial parietal cortex, and progressive atrophy when serial scans are available.There are 2 types of PET biomarkers: those that show amyloid deposition, and those that show neurodegeneration. Brain neurodegeneration can be identified by measuring glucose metabolism in the brain with FDG PET. These scans show which areas of the brain are and are not functioning well. Decreased metabolism is observed in temporal and parietal cortex when the AD pathophysiological process has caused neurodegeneration.[8] FDG PET scans are available to the clinician now, and are covered by Medicare. We do not, however, recommend using these scans routinely when the history, physical examination, cognitive testing, and structural imaging are all consistent with AD—it simply is not necessary.[8] In situations, however, in which one suspects an atypical neurodegenerative disease, an FDG PET scan can help distinguish AD from another disorder (such as dementia with Lewy bodies or frontotemporal dementia). Another reason to obtain an FDG PET scan is if one is suspecting AD in a patient younger than 66 years of age. At such a young age the prevalence of AD is similar to that of many other etiologies (such as frontotemporal dementia),[9] making it more critical to be sure that the diagnosis is AD before treatment is initiated.Several compounds that can identify AB deposition using PET are currently being used for research and/or are under commercial development. The 2 most widely used are the Pittsburgh compound B and florbetapir. Both have been shown to bind to brain AB.[10,11] Because amyloid accumulation is thought to be present years before the onset of clinical symptoms, PET identification of AB raises the possibility of identifying and treating patients with AD years before symptoms emerge—once a drug has been proven to be disease modifying. Amyloid PET imaging is currently being used in several clinical trials both to help identify individuals with preclinical AD and to measure the effects of antiamyloid treatments. Moreover, florbetapir has recently been submitted to the FDA for approval in clinical practice. Detection of β-amyloid by PET could be available to clinicians soon.Standard CSF biomarkers for AD are Aβ42, total tau, and p-tau. Studies have shown that when all 3 markers are combined, the accuracy of the diagnosis is highest, with sensitivity and specificity of 85% to 90%.[12] Although CSF analysis is already commercially available (www.athenadiagnostics.com) and used by some clinicians to aid in diagnosis, we view this test as highly promising but not yet ready for routine clinical practice.[8]Based upon the current scientific model regarding when different events occur in the AD pathophysiological process (Fig. 4), Sperling et al[2] discussed 3 stages of preclinical AD to be considered for research, based upon biomarkers and cognitive change. Stage 1 is asymptomatic cerebral amyloidosis, which could be determined by the presence of a biomarker sensitive to Aβ, in the absence of any marker of neurodegeneration or evidence of subtle cognitive change. Stage 2 is asymptomatic cerebral amyloidosis plus neurodegeneration in the absence of subtle cognitive change. Finally, stage 3 is amyloidosis plus neurodegeneration plus subtle cognitive or behavioral decline. Guided by these theoretical underpinnings, the new clinical and research criteria are next presented for MCI due to AD and AD dementia.
Criteria for Mild Cognitive Impairment Due to the AD Pathophysiological Process
Based upon guidelines currently in use at memory centers and in research studies, Albert et al[3] presented the criteria for MCI due to AD. These criteria, aimed both at clinicians and researchers, refer to the symptomatic phase of the AD pathophysiological process but before the individual develops the functional impairment that defines dementia. Clinicians are increasingly making a diagnosis of MCI and the new diagnostic guidelines will formalize this process. The guidelines recognize that everyone who eventually develops AD will go through a transitional period of mild but detectable cognitive impairment. Importantly, however, not everyone who is diagnosed with MCI will go on to develop AD. MCI can be due to a variety of disorders including, vascular dementia, frontotemporal dementia, dementia with Lewy bodies, and others. Both core clinical criteria for the diagnosis of MCI due to AD and clinical research criteria are presented. As the authors start their discussion on the core clinical criteria they point out that, in addition to these criteria, clinical judgment must be used to distinguish between normal cognition and MCI, and between MCI and dementia.
The new guidelines specify the criteria for making a diagnosis of MCI due to AD. The criteria include:
- Excluding patients who have other causes of MCI including extensive vascular disease, frontotemporal dementia, and dementia with Lewy bodies. These exclusions will likely be accomplished through a combination of the clinical evaluation and imaging studies.
- Including patients who show increasing cognitive decline over time. This decline could be detected historically or via serial evaluations over time.
- Including patients who have the very rare mutations associated with early-onset familial AD (amyloid precursor protein, presenilin 1, or presenilin 2). These mutations can be identified via commercially available genotyping (http://www.athenadiagnostics.com).
Because patients with MCI due to AD may actually be considered very early AD patients, it will be important for practicing clinicians to identify this condition. There may be at least as many patients with MCI due to AD as there are with dementia due to AD.[13,14] Although there is not substantial evidence that current treatments with FDA approved medications improve patients with MCI due to AD (there is ongoing research aimed at answering this question), patients with this condition may benefit from referrals to centers conducting studies with potential disease modifying treatments such as vaccines that are aimed at slowing the loss of neurons and thus the progression of the disease. It is becoming increasingly clear that disease-modifying drugs will be most beneficial in the earliest stages of the disease. There are now several ongoing studies with hypothesized disease modifying agents (eg, vaccines) for patients with MCI due to AD, and other studies are being planned.
The clinical criteria are divided into 2 parts (Table 2). First, criteria are presented for the clinical and cognitive syndrome of MCI, and second, criteria are presented regarding the etiology of the MCI syndrome being consistent with AD.
Research criteria for MCI due to AD incorporating biomarkers are next presented. The biomarkers discussed include those in Table 1. Two valuable reasons to add biomarkers is that they may help determine (1) the underlying etiology of the MCI and (2) the likelihood and time course of an individual with MCI becoming more impaired or demented. An important point discussed is that biomarkers that detect Aβ protein deposition are most useful in determining underlying etiology, whereas biomarkers that detect neuronal degeneration and injury are most useful in determining prognosis and progression. Adding each of these categories of biomarkers to the core clinical diagnosis of MCI due to AD leads to the following probabilities: If 1 of these 2 biomarker categories is positive, the "biomarker probability of AD etiology" rises to "intermediate," and both categories must be positive for the "highest" probability. The "lowest" probability is present if both categories are negative.
Criteria for All-cause Dementia and AD
The new criteria for AD clarify existing guidelines and are appropriate for use today by practicing physicians. The new guidelines propose 4 possible classifications of dementia caused by AD: (1) probable AD dementia, (2) probable AD dementia with increased level of certainty, (3) possible AD dementia, and (4) probable or possible AD dementia with evidence of AD pathophysiological process. The last criteria propose a system for providing additional diagnostic evidence through the use of biomarkers, and are currently intended for research purposes only, although it is likely that it will be increasingly used in future clinical practice. The hope is that the use of these new clinical criteria coupled with the judicious addition of biomarkers will eventually improve diagnostic accuracy and help make accurate diagnoses earlier in the disease process when intervention can be most beneficial. The new guidelines do not suggest any additional diagnostic procedures for patients already diagnosed with AD.
The new criteria suggest a 4-step approach to diagnosis of dementia due to AD (Table 3). Step 1 determines that dementia is present, step 2 determines that the dementia is due to AD, step 3 provides an increased level of certainty to the diagnosis, and step 4 evaluates the biomarker probability of AD etiology.
The next idea that is introduced is that of research criteria for "probable AD dementia with evidence of the AD pathophysiological process." These research criteria increase the certainty that the patient's clinical dementia is due to the AD pathophysiological process by adding biomarker evidence to the probable AD dementia criteria. The biomarkers fall into 2 categories, markers of Aβ protein deposition in the brain and markers of downstream neurodegeneration, (Table 1).
If 1 of these 2 biomarker categories is positive, the "biomarker probability of AD etiology" rises to "intermediate," and if both categories are positive the probability becomes "high." The authors are specific that they do not advocate obtaining AD biomarkers for routine clinical purposes at the present time, although they do note that they may be used when they are available and deemed appropriate by the clinician.
The clinical criteria for possible AD dementia can be found in Table 4. The term "possible" is used instead of "probable" in 2 scenarios. One is if the cognitive deficits look like AD but there is an atypical course of the disease: either sudden onset or no definite decline. The second is if there is evidence for a mixed etiology of the dementia, such that the patient meets criteria for probable AD dementia but there is also evidence of significant vascular disease, features of dementia with Lewy bodies, or other disease or condition that could be contributing to the patient's dementia.
Pathophysiologically proven AD is next discussed, consisting simply of the unchanged criteria of patients meeting both the clinical and neuropathologic criteria for AD. Finally the criteria for dementia unlikely to be due to AD is discussed (Table 5).
Implications for the Practicing Clinician
These new guidelines do not suggest a fundamental difference in the way clinicians will diagnose AD dementia or MCI due to AD. For now, diagnosis of these disorders will continue to be primarily clinical. The new guidelines also do not recommend any specific treatment strategies. Implicitly or explicitly, however, they do encourage clinicians to:
- Recognize that AD is the end of a long process, spanning years or perhaps decades, leading to death of brain cells, cognitive loss, and ultimately dementia.
- Diagnose (and perhaps treat) AD at the earliest possible stage. At present this would be the MCI due to AD stage, but eventually will include preclinical AD.
- Begin to consider using biomarkers in the diagnosis of all stages of AD. For now, most biomarkers will only be used in research studies (both for diagnostic purposes and to evaluate the efficacy of disease modifying treatments), but some either are available to clinicians now (eg, FDG PET scans, CSF Aβ42, and tau) or will be in the near future (eg, quantitative MRI, amyloid PET scans).
- Evaluate patients with dementia to determine the cause of the dementia with special attention paid to amnestic and nonamnestic presentations of AD.
- Remember that they will soon see a significant increase in patients in their practices with AD. There are currently 5.4 million patients in the United States with AD dementia and perhaps an equal amount with MCI. Because of the aging population, these numbers could triple in the next 50 years.
Conclusions
Today, these new criteria allow the practicing clinician to diagnose AD dementia and MCI due to AD with more clarity, providing greater certainty of the diagnosis for patients and families. In the future, these criteria will provide practitioners with the tools they need to know which patients are appropriate for the disease-modifying medications which are being developed to slow or even stop the AD pathophysiological process.


No hay comentarios:
Publicar un comentario